Exscientia is now Recursion
Stay up to date at www.Recursion.com
Pipeline
Our pipeline demonstrates the ability of our AI-driven drug discovery platform to create precision engineered, patient-centric therapeutic candidates.
Programme
Target
Late Discovery
IND / CTA- Enabling
Phase 1/2
Pivotal / Approved
Exscientia Rights
GTAEXS617

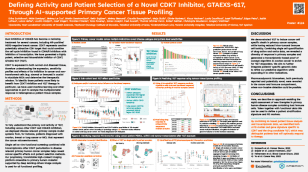
GTAEXS617 (‘617) (CDK7 inhibitor):A Phase 1/2 trial to evaluate ‘617 for the treatment of solid tumours is enrolling patients.
Inhibiting CDK7 presents an opportunity to improve treatment outcomes over CDK4/6 inhibitors due to CDK7’s dual role in cell cycle and transcription. Previous development efforts have exhibited side effects, possibly due to a covalent binding mechanism of action or poor oral absorption.
We were able to identify a molecule meeting all of our design criteria after testing just 136 compounds. Our selective, non-covalent clinical candidate meets multiple criteria, including high on-target potency and selectivity with expected improved absorption over competitors. ‘617 also has favourable preclinical oral bioavailability and, critically, it demonstrates a significantly reduced interaction with the key efflux transporter P-gp compared to other CDK7 candidates in development.
In preclinical studies, ‘617 delivered a strong in vivo anti-tumour profile as demonstrated in both ovarian and triple negative breast cancer xenograft models.
Using our precision medicine platform, we are working to:
- define and understand activity in more than six solid tumour indications,
- discover and qualify pharmacodynamic markers and
- explore methods to enrich for responding and non-responding patients.
These will be used and confirmed alongside our Phase 1/2 trial.
EXS4318

EXS4318 (‘4318) (PKC-theta inhibitor): ‘4318 has been in-licensed by Bristol Myers Squibb (BMY), who announced in February 2023 that ‘4318 has entered a Phase 1 clinical trial in the U.S. Positive early Phase 1 results were announced in May 2024.
PKC-theta plays a critical role in controlling T-cell function and is a key driver of several highly prevalent autoimmune diseases, therefore providing an attractive immune modulating drug target.
PKC-theta inhibitors have potential in inflammatory and immunologic diseases; however, several large pharma companies have failed to design a small molecule with the required potency and selectivity against other closely related kinases. Exscientia’s platform designed a highly potent, highly selective next-generation immunomodulatory drug candidate within 11 months of starting the design process; it was the 150th molecule synthesised.
The human dose prediction for ‘4318, which is calculated by a composite of numerous pharmacological properties (including cross-species PK and potency), is very favourable. ‘4318 is a balanced candidate that has demonstrated high on-target activity while maintaining high selectivity and favourable tolerability.
EXS74539

EXS74539 (‘539) (LSD1 inhibitor): IND submission expected in 2024.
‘539 is the first potent, selective, reversible and brain penetrant LSD1 inhibitor with potential in both haematology and solid tumours. LSD1 demethylates histones, which play a critical role in regulating the expression of genes that suppress differentiation and drive the proliferation and survival of several tumour types.
To date, other LSD1 inhibitors in development have failed to achieve the combination of appropriate pharmacokinetics, good brain penetrance and a reversible mechanism of action. Exscientia’s candidate, ‘539, achieves a design objective of suitable CNS penetration to target brain metastases, which are prevalent in certain cancer subtypes.
Evaluation of ‘539 using our precision medicine platform and through in vivo studies has shown favourable activity in small-cell lung cancer (SCLC), with dose dependent inhibition of tumour growth observed in SCLC xenograft models. Studies have also shown a favourable absorption, distribution, metabolism and excretion (ADME) profile, with a shorter predicted human half-life than some LSD1 inhibitors currently in clinical trials – this potentially enables a better therapeutic index. No safety concerns have been observed in preclinical studies conducted to date.
EXS73565

EXS73565 (‘565) (MALT1 protease inhibitor): CTA submission expected in 2024.
‘565 is a potent and selective MALT1 protease inhibitor with potential applications in haematology. MALT1 is a protease crucial for activation of the NF-κB pathway, which supports the uncontrolled proliferation of malignant T- and B-cells in haematological cancers.
Exscientia’s precision design approach optimised the safety profile for agents targeting MALT1 whilst also generating potency and selectivity. Scaffolds of other MALT1 inhibitors in the clinic significantly inhibit UGT1A1, an enzyme involved in the metabolism of bilirubin, often leading to dose-limiting toxicities in humans.
In vivo studies of ‘565 have shown anti-tumour activity in murine models and favourable pharmacokinetics both as monotherapy and in combination with ibrutinib. Toxicology studies have shown that ‘565 has an acceptable therapeutic index, with the ability to maintain high levels of potency, selectivity and safety benchmarks, while avoiding meaningful inhibition of UGT1A1, which can lead to hyperbilirubinemia.
PKC-theta is in a Phase 1 healthy volunteer (HV) study; AML = acute myeloid leukaemia; SCLC = small-cell lung cancer